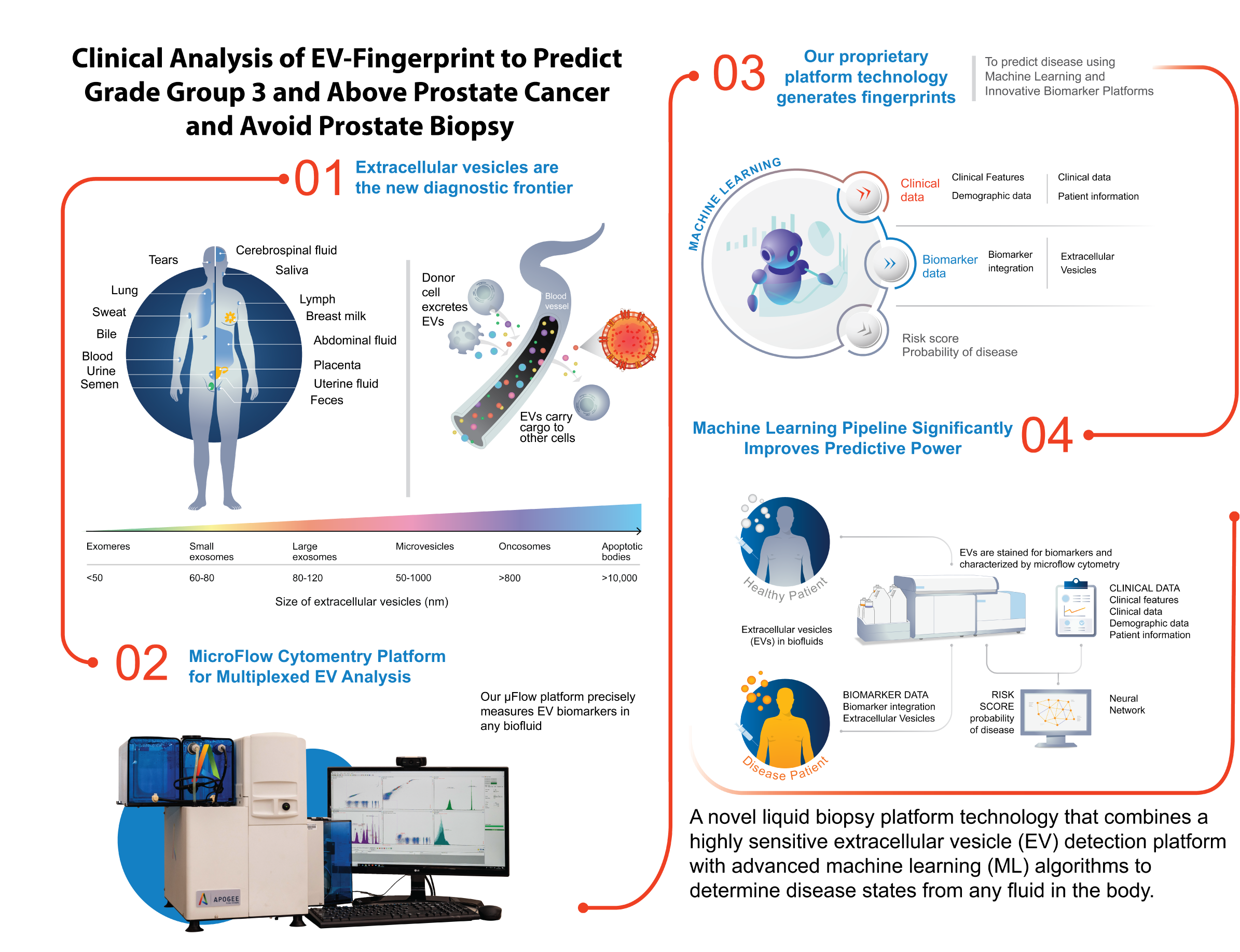
Nanostics Launches ClarityDX Prostate Test in Alberta to Significantly Improve Prostate Cancer Screening
perrin2024-01-10T22:12:10+00:00EDMONTON, ALBERTA – September 28 – Nanostics is thrilled to announce the availability of the ClarityDX Prostate test at its newly accredited clinical lab in Edmonton, Alberta. This innovative blood test marks a significant advancement in prostate cancer screening. It provides critical support to men, aged 40 to 75, and their physicians in making more informed decisions, with 3X the accuracy, about whether a biopsy is required following a high prostate-specific antigen (PSA) test result.
According to Dr. John D. Lewis, CEO of Nanostics and Bird Dogs Chair of Translational Oncology at the University of Alberta, “This test will reduce the number of unnecessary prostate biopsies, which are invasive, uncomfortable, and carry some risk.” Notably, research shows that adding ClarityDX Prostate to the patient care pathway could reduce unnecessary prostate biopsies by up to 35% and yield significant cost savings.
Click below to read the full press release.