Our core technology can
- Identify biomarkers expressed by diseased cells/tissues
- Indicate if treatment is needed
- Predict treatment response and resistance
- Stratify disease stage
- Identify disease recurrence

The ClarityDX Prostate test can help physicians and patients navigate biopsydecision at TWO critical points in care
Initial diagnosis:
ClarityDX Prostate is designed to accurately predict the presence of clinically significant prostate cancerand with the guidance of a physician can be used to inform the decision to biopsy.
Active surveillance:
ClarityDX Prostate is currently under evaluation as a test to monitor patients on active surveillance to detect theprogression to clinically significant prostate cancer and inform the decision to biopsy.

The ClarityDX Prostate test was validated in multiple studies in partnership with APCaRI and DynaLIFE.
The test achieved accuracy measurements of > 0.82 AUC (Area Under the Curve) for the identification of clinically significant prostate cancer, compared to 0.58 AUC for the current standard of care Prostate-Specific Antigen (PSA) test.
ClarityDX Prostate was found to be up to 3X more accurate than PSA.
ClarityDX Prostate
Prostate cancer blood test.
Sector
Diagnostics and Liquid Biopsy.
Platform
Developed with the ClarityDX Machine Learning Analysis Platform.
Clinical Studies
AUC: > 0.82
Sensitivity: 95%
NPV: 91%
Accurate Diagnosis
Our test detects clinically significant prostate cancer with the highest accuracy in prospective clinical studies compared to similar tests in the market.
High Impact
ClarityDX Prostate could eliminate up to 600,000 unnecessary biopsies and 24,000 related hospitalizations in North America each year.
Clarity in healthcare decisions
By differentiating between indolent and clinically significant prostate cancer, ClarityDX Prostate can guide physicians and patients through the decision process. As a result, men may be spared the adverse events of biopsy, prostate cancer surgery, radiotherapy, and hormone therapy.
Cost-effective
ClarityDX Prostate will save an estimated $1.4 billion in healthcare costs by reducing the number of prostate biopsies and unnecessary treatments such as surgery and radiation.
Clinical utility
ClarityDX Prostate is a non-invasive and easily performed test, ideal for large-scale population-based screening in medical clinics, worldwide.
Clinical Studies
A clinical validation study (Abstract MP 9.8 Paproski et al. 2023. CUAJ. pS117-8) with 3,448 men, including 1,409 men from Alberta, demonstrates that ClarityDX Prostate is 3X more specific than PSA at detecting clinically significant prostate cancer. This improved specificity can be used to better inform clinical decision-making on whether to proceed to prostate biopsy.
Nanostics received CPSA accreditation to provide the ClarityDX Prostate test to patients through its clinical testing lab in Edmonton, Alberta. Nanostics is currently preparing further approvals through the US FDA.
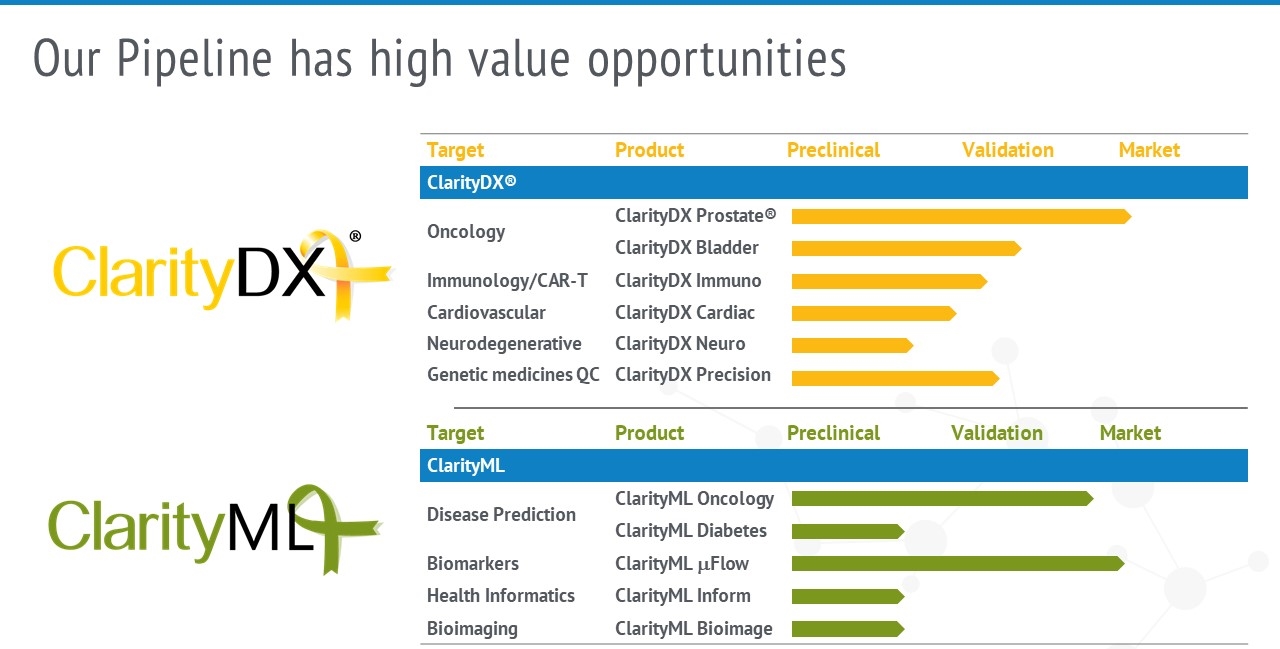
New Applications and Product Pipeline
The ClarityDX platform technology is well-positioned for future pipeline products including tests for screening, early diagnosis, complementary diagnosis, monitoring, and management of many diseases and medically related events.
Nanostics’ R&D team continues to expand the product pipeline through in-house and partnered projects to include additional liquid biopsy diagnostic tests to improve patient care.
Development of the ClarityDX platform for use with many disease targets.