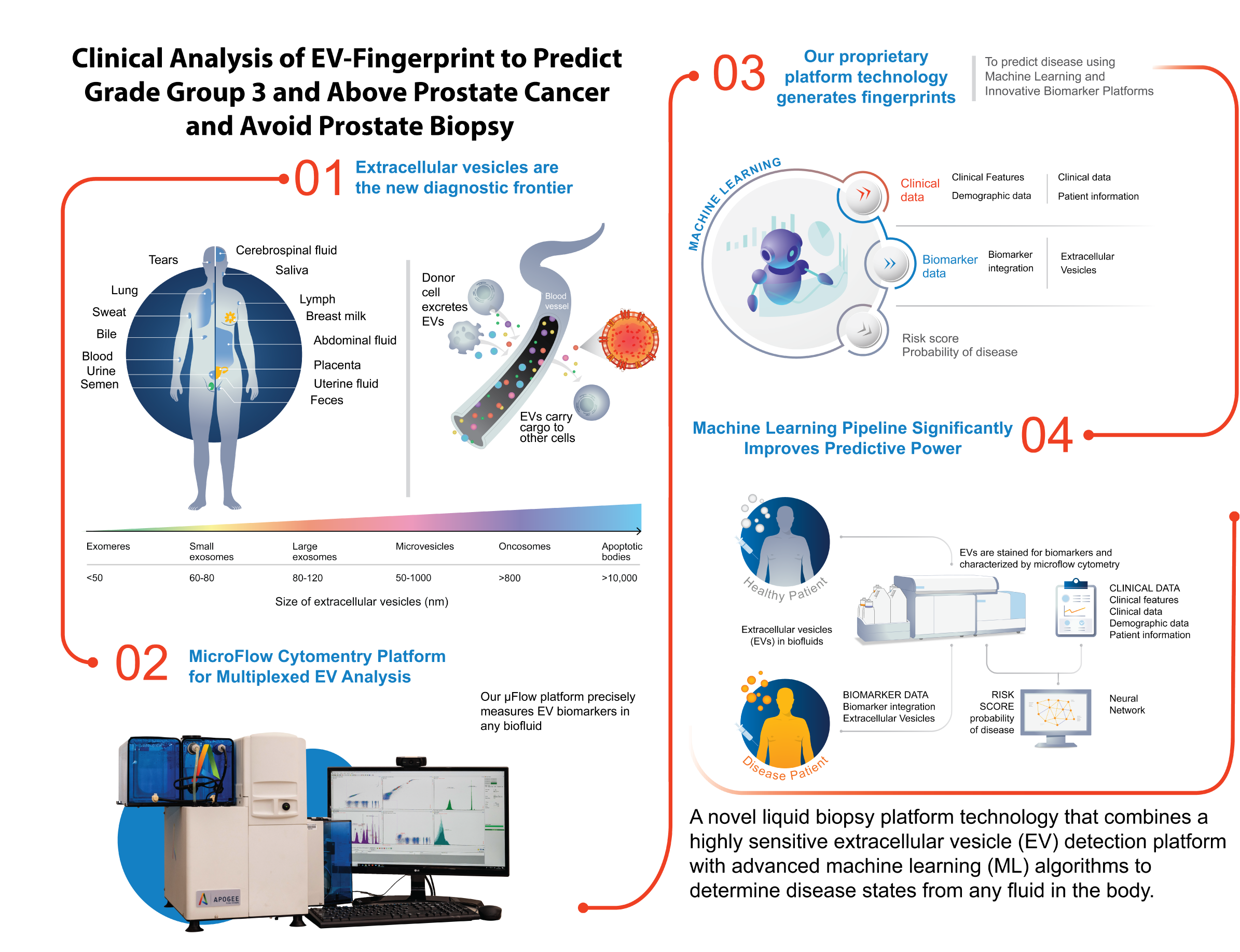
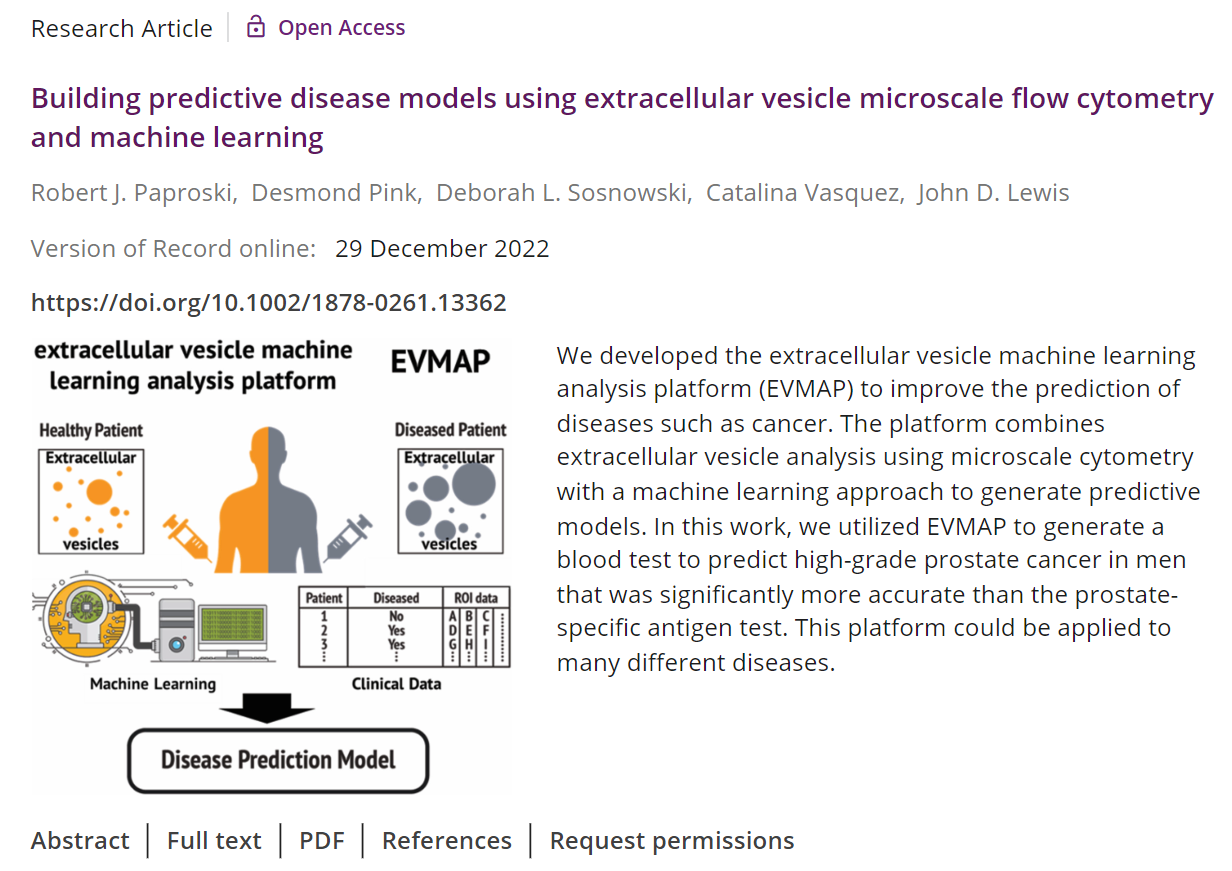
Research supporting ClarityDX Prostate published in Nature Digital Medicine
Natalia Hotsaliuk2024-07-03T19:51:25+00:00Development of an effective predictive screening tool for prostate cancer using the ClarityDX machine learning platform
M. Eric Hyndman, Robert J. Paproski, Adam Kinnaird, Adrian Fairey, Leonard Marks, Christian P. Pavlovich, Sean A. Fletcher, Roman Zachoval, Vanda Adamcova, Jiri Stejskal, Armen Aprikian, Christopher J. D. Wallis, Desmond Pink, Catalina Vasquez, Perrin H. Beatty & John D. Lewis